Specific Radioactive Elements and Their Effects on Health.
From Hiroshima to Fukushima to You, Dale Dewar, 4 July 2024 “……………………………………………………………………………………………………………..
That radioactive elements cause cancer is beyond doubt. Increasing their presence in our environment does increase the incidence of cancer. It seems that these elements may cause any number of other problems – auto-immune and cardiovascular diseases, ill-health and chronic tiredness, headaches and benign tumours all have suspicious links. Lowered resistance to bacterial and viral illnesses has been seen.
Funding to do the studies that extend over years is not available.
Even an accident as large as the Three Mile Nuclear Power plant accident received funding for only nine years. When studies done by Joseph Mancuso, Alice Stewart and Geoffry Knean on Hanford workers showed a health effect not only was their funding cut but demands were made that they release all their hard data to the National Research Council. (Mancuso lost his data but Stewart and Knean had taken most of the documentation home with them, to the UK.)
That radioactivity causes chromosomal defects in fruit flies is also not questioned. To show these effects, if they occur in humans, would require centuries.
The specific effects of some radioactive elements have been well studied:
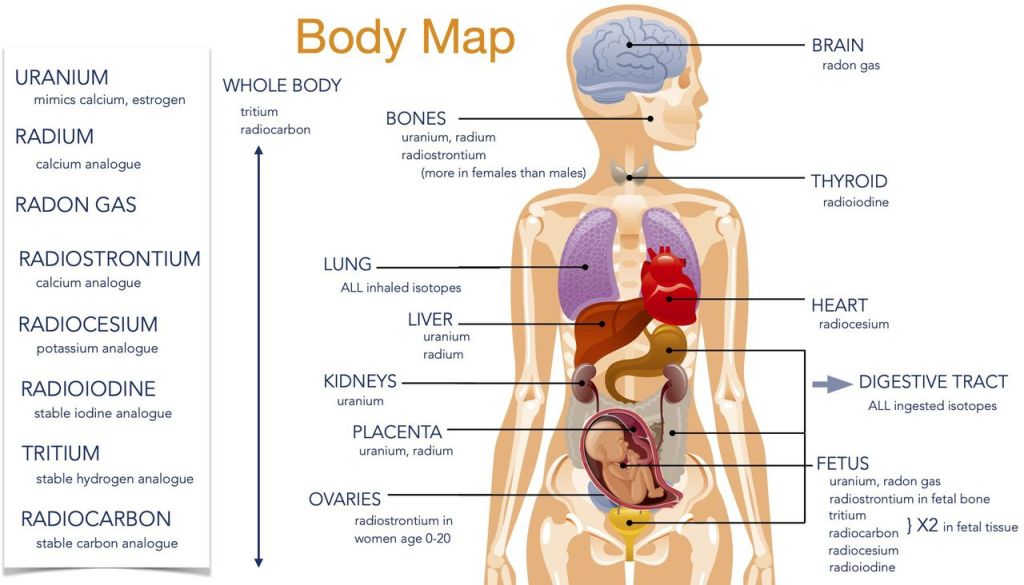
Radon-222: Cancers caused by radon prompted the Canadian government to establish the Canadian National Radon Program using guidelines developed by the International Radiation Protection Association. Various public health offices believe that alpha radiation from radon causes up to 20% of Canadian lung cancers.
Radon is the main decay product of radium. It has a half-life of only 3.8 days so its decay chain is also of concern for health. One of its products is polonium-210, one of the most poisonous elements on earth. Are cancers blamed on radon really caused by polonium?
Radon has found some use as a tracer but, while found naturally, it is still considered part of uranium waste.
Uranium-238: This isotope of uranium is its most common. Forming 99.27% of natural uranium, it has a half-life of 4.5 billion years. It is the starting of a decay chain that includes radium, radon, polonium and ends with stable lead-210. This isotope, uranium-238, is popularly referred to as “depleted uranium” because its uranium-235 has been removed.
Uranium is a heavy metal and as such, its health effects resemble those of lead and mercury, kidney failure being the most common. It seems to have estrogen-mimicking properties and at least one chronic disease has found to be increased, systemic lupus erythematosus, among a cohort of uranium miners.
The Eldorado uranium miners study looked specifically for lung cancer and found a doubling effect – but was it due to powdered uranium or gaseous radon?
Uranium-235: This isotope is fissile, the isotope desired for nuclear bombs. “Enrichment” of uranium occurs to increase the percentage of U-235 and there are various percentages required for different tasks.
Most light water nuclear reactors require a concentration of 3 – 5% U-235 to operate, to reach criticality and produce the heat to boil water. It is anticipated that the proposed small modular reactors will require HALEU (High Assay Low Enriched) uranium which contains 19.5% uranium-235.
Aside from nuclear bombs and nuclear power plant fuel, uranium has no other functions. Uranium as an ore, refined to “yellow cake” is not very radioactive.
Radium-226: The most stable isotope of radium with a half-life of 1600 years is radium 226, itself a decay product of thorium-230 in the uranium-238 decay chain. Radium is considered the most radioactive element known. It emits alpha, beta and gamma radiation. Its glowing colour is the result of ionization of the air around it.
All 34 known isotopes are radioactive. It is found in nature.
Radium’s use has evolved from the dials of watches until the 1970’s and cancer treatments until the 1990’s when it was discarded in favour of less radioactive but still effective elements. It may have been the first element used in brachytherapy where the element is encapsulated and inserted inside a tumour. It is still used for prostate cancer that has spread to bones.
Radium is a relative of calcium and strontium. When it is in the blood, bones and muscles will absorb it and use it in place of calcium. In the bones and muscles, its radiation induces bone cancers, and cancers of the bone marrow (leukemias). Hence the dial workers and the industrialist developed bone cancers, osteosarcomas.
Strontium-90: Strontium (element 38) is found ubiquitously in radioactive fallout from nuclear bombs or nuclear power plants. It is a fission product of uranium.
Natural strontium is not radioactive, nor are its four isotopes. It belongs to the same family of elements as calcium and human biology treats them very similarly, strontium is scooped out of the blood to incorporate it into bones and muscles. It is believed to have a biophysical[4] half-life of 18 years. Because it is very close to blood-forming components in the bones, it is blamed for increases in leukemia, lymphomas and bone cancers. While in situ, it initially weakens bones.
Strontium-90 decays with a half-life of 29 years to yttrium-90 which also undergoes beta decay to zirconium-90 which is stable.
Strontium-90 has no commercial value and is considered entirely an environmental pollutant.
Iodine-131: Radioactive iodine therapy increases the risk of leukemia, stomach cancer and salivary gland cancer, according to the American Cancer Society[xxiii]. On March 27, 2011, Massachusetts Department of Public Health found I-131 in low concentration in rain water, likely originating from the Fukushima accident.
Iodine-127 is the only stable isotope of the element with 53 protons in its nucleus. Of the remaining 26 isotopes, iodine-131 is not only of greatest concern with respect to nuclear bomb testing fallout, nuclear power plant accidents and natural gas production, but of all fission-related radioisotopes, it has also found the greatest medical use. It has a half-life of about 8 days and emits an energetic electron, a beta particle. It is preferentially filtered out of the blood by the thyroid.
Because it is collected by the thyroid, it can be used in high doses to selectively kill hyperactive thyroid cells whether they are benign or malignant. Also, because it is collected by the thyroid, its action can be mitigated by taking normal oral iodine at the time of exposure.
Its short half-life means that it is an insignificant contributor to nuclear waste.
It decays to xenon-131 which is stable.
Tritium: All threehydrogen isotopes are gasses and can form water with oxygen. Hydrogen itself has one proton in its nucleus and one electron circling it. Deuterium is “heavy water” with one proton and one neutron in its nucleus. Tritium is radioactive with one proton and two neutrons in its nucleus.
While it is naturally formed by cosmic rays hitting hydrogen in the upper atmosphere, the bulk of today’s tritium is released from nuclear power plants. It is often characterized as a short-lived weakly radioactive radioisotope, but a half-life of 12.3 years is questionably “short” in human terms. The beta particle emitted by tritium is low energy but its presence inside human cells is a major concern.
Getting into human cells is pretty easy for a hydrogen isotope because, combined with oxygen, it forms tritiated water and water enters every cell of almost every biological being. It is very difficult to link specific exposures to cancers and chronic disease but using populations studies, researchers can link the health of populations around nuclear power plants with case-matched[5]populations that are not exposed to tritium releases from power plants.
Tritium has had commercial use as the energy source in radio luminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains[xxiv]. It is used in a medical and scientific setting as a radioactive tracer. The past use in exit signs was discontinued because of breakage.
Conclusion:
Does ionizing radiation cause cancer? Cancer seems to be at least one consequence of exposure. While it is difficult to determine whether a person has developed cancer because he/she worked in a uranium mine, had a high amount of radon in their home, got struck by cosmic rays, or had too much glyphosate or benzo(a)pyrene[6] in their diet, wherever the more difficult comparison of populations has been done, those affected by the higher ionizing radiation regardless of the element, show increased incidences of cancer.
We can say with certainty is that ionizing radiation causes ions. When It enters human cells, it can pass straight through or, like a cyclone, wreak havoc on the cell’s internal structure.
Ionizing radiation can break up chromosomes, the things in cells that tell the cell what it is. If it is a skin cell, the chromosome will tell the cell to make more skin cells. If the chromosome has been damaged, it may not be able to tell the cell how to make normal skin cells.
To say that ionizing radiation is safe is fraudulent.
What can you do to limit your exposure to ionizing radiation?
1. Whenever you or a child or someone under your care is asked to have an x-ray, ask the person ordering it how the x-ray result will change or otherwise affect treatment. Often the answer will be that they simply want to assess your progress. If you feel good (or better), you already know your progress.
2. Make sure that you are getting the right imaging for the problem you are facing. When a CT scan was suggested for one of my patients, I realized that he would be better served by an MRI which then revealed the small cyst in a tendon.
3. Don’t succumb to the doctor or other care provider’s “curiosity”. Ask questions.
My patient, call him “John”, told me this story. At 79 years of age, he had Chronic Myelogenous Lymphoma and was told by his specialist to have a biannual CT scan. He was feeling quite well.
He asked the doctor, “What are you looking for?” He was told that the physician was looking for “changes”. John already had one CT scan and hadn’t been told the results.
The specialist said that he hadn’t mentioned the previous CT scan because there wasn’t much to report. John thanked him and refused the new CT scan. He told the specialist he would return if his health changed.
4. There is almost no excuse for “routine x-rays”. At one time everyone who entered a hospital was submitted to chest x-rays.
To these choices that affect you personally, there is another action that we should be taking:
5. Oppose development of nuclear weapons and nuclear power. One will not exist without the other. While medical radioisotopes don’t need nuclear power reactors for their use and development nuclear bombs cannot be built or serviced without nuclear power. _…………………….. https://ionizingradiationandyou.blogspot.com/
No comments yet.
-
Archives
- January 2026 (288)
- December 2025 (358)
- November 2025 (359)
- October 2025 (376)
- September 2025 (258)
- August 2025 (319)
- July 2025 (230)
- June 2025 (348)
- May 2025 (261)
- April 2025 (305)
- March 2025 (319)
- February 2025 (234)
-
Categories
- 1
- 1 NUCLEAR ISSUES
- business and costs
- climate change
- culture and arts
- ENERGY
- environment
- health
- history
- indigenous issues
- Legal
- marketing of nuclear
- media
- opposition to nuclear
- PERSONAL STORIES
- politics
- politics international
- Religion and ethics
- safety
- secrets,lies and civil liberties
- spinbuster
- technology
- Uranium
- wastes
- weapons and war
- Women
- 2 WORLD
- ACTION
- AFRICA
- Atrocities
- AUSTRALIA
- Christina's notes
- Christina's themes
- culture and arts
- Events
- Fuk 2022
- Fuk 2023
- Fukushima 2017
- Fukushima 2018
- fukushima 2019
- Fukushima 2020
- Fukushima 2021
- general
- global warming
- Humour (God we need it)
- Nuclear
- RARE EARTHS
- Reference
- resources – print
- Resources -audiovicual
- Weekly Newsletter
- World
- World Nuclear
- YouTube
-
RSS
Entries RSS
Comments RSS





Leave a comment